Surgical gowns are regulated by the FDA as a Class II medical device and requires a 510(k) premarket notification. A surgical gown is a personal protective garment intended to be worn by health care personnel during surgical procedures to protect both the patient and health care personnel from the transfer of microorganisms, body fluids, and particulate matter. Because of the controlled nature of surgical procedures, critical zones of protection have been described by national standards.
Medical Gowns
Critical zones include the front of the body from top of shoulders to knees and the arms from the wrist cuff to above the elbow. Surgical gowns can be used for any risk level (Levels 1-4). All surgical gowns must be labeled as a surgical gown.
In 2004, the FDA recognized the consensus standard American National Standards Institute/Association of the Advancement of Medical Instrumentation (ANSI/AAMI) PB70:2003, “Liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities.” New terminology in the standard describes the barrier protection levels of gowns and other protective apparel intended for use in health care facilities and specifies test methods and performance results necessary to verify and validate that the gown provides the newly defined levels of protection:
- Level 1: Minimal risk, to be used, for example, during basic care, standard isolation, cover gown for visitors, or in a standard medical unit
- Level 2: Low risk, to be used, for example, during blood draw, suturing, in the Intensive Care Unit (ICU), or a pathology lab
- Level 3: Moderate risk, to be used, for example, during arterial blood draw, inserting an Intravenous (IV) line, in the Emergency Room, or for trauma cases
- Level 4: High risk, to be used, for example, during long, fluid intense procedures, surgery, when pathogen resistance is needed or infectious diseases are suspected (non-airborne)
Regardless of how the product is named (that is, isolation gown, procedure gown, or cover gown), when choosing gowns, look for product labeling that describes an intended use with the desired level of protection based on the above risk levels. Product names are not standardized.
Surgical Gowns
A surgical gown is regulated by the FDA as a Class II medical device that requires a 510(k) premarket notification. A surgical gown is a personal protective garment intended to be worn by health care personnel during surgical procedures to protect both the patient and health care personnel from the transfer of microorganisms, body fluids, and particulate matter. Because of the controlled nature of surgical procedures, critical zones of protection have been described by national standards. As referenced in Figure 1: the critical zones include the front of the body from top of shoulders to knees and the arms from the wrist cuff to above the elbow. Surgical gowns can be used for any risk level (Levels 1-4). All surgical gowns must be labeled as a surgical gown.
Surgical Isolation Gowns
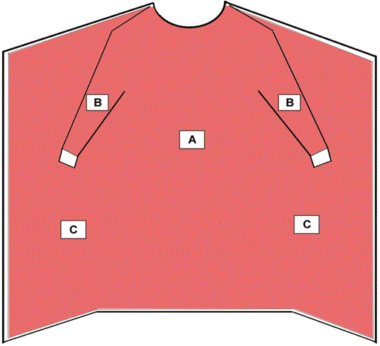
Surgical isolation gowns are used when there is a medium to high risk of contamination and a need for larger critical zones than traditional surgical gowns. Surgical isolation gowns, like surgical gowns, are regulated by the FDA as a Class II medical device that requires a 510(k) premarket notification. As referenced in Figure 2, all areas of the surgical isolation gown except bindings, cuffs, and hems are considered critical zones of protection and must meet the highest liquid barrier protection level for which the gown is rated. All seams must have the same liquid barrier protection as the rest of the gown. Additionally, the fabric of the surgical isolation gown should cover as much of the body as is appropriate for the intended use.
Critical Zones for Surgical Isolation Gowns and Non-Surgical Gowns
- The entire gown (areas A, B, and C), including seams but excluding cuff, hems, and bindings, is required to have a barrier performance of at least Level 1.
- Surgical isolation gowns are used when there is a medium to high risk of contamination and need for larger critical zones than traditional surgical gowns.
Non-Surgical Gowns
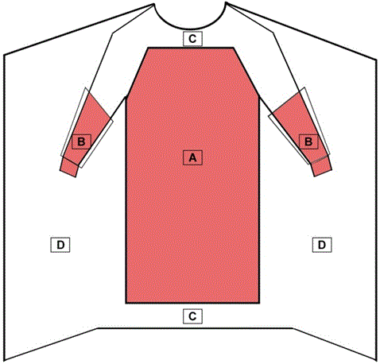
Non-surgical gowns are Class I devices (exempt from premarket review) intended to protect the wearer from the transfer of microorganisms and body fluids in low or minimal risk patient isolation situations. Non-surgical gowns are not worn during surgical procedures, invasive procedures, or when there is a medium to high risk of contamination.
Like surgical isolation gowns, non-surgical gowns should also cover as much of the body as is appropriate to the task. As referenced in Figure 2, all areas of the non-surgical gown except bindings, cuffs, and hems are considered critical zones of protection and must meet the highest liquid barrier protection level for which the gown is rated. All seams must have the same liquid barrier protection as the rest of the gown.
Critical Zones for Non-Surgical Gowns
- The entire front of the gown (areas A, B, and C) is required to have a barrier performance of at least level 1.
- The critical zone compromises at least areas A and B.
- The back of the surgical gown (area D) may be non-protective.
Standards for Gowns
Labeling that shows a product has been tested to and meets appropriate performance standards is one way for users and procurers to determine when to use a particular gown.
The performance of gowns is tested using consensus standards:
American Society for Testing and Materials (ASTM) F2407 is an umbrella document, which describes testing for surgical gowns: tear resistance, seam strength, lint generation, evaporative resistance, and water vapor transmission.
Below is a summary of ASTM F2407 standard recognized by the FDA.
- Tensile Strength: ASTM D5034, ASTM D1682
- Tear resistance: ASTM D5587(woven), ASTM D5587 (nonwoven), ASTM D1424
- Seam Strength: ASTM D751 (stretch woven or knit)
- Lint Generation (ISO 9073 Part 10)
- Water vapor transmission (breathability) ASTM F1868 Part B, ASTM D6701 (nonwoven), ASTM D737-75
American National Standards Institute (ANSI) and the Association of the Advancement of Medical Instrumentation (AAMI): ANSI/AAMI PB70:2003 describes liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities.
Below is a table summarizing the ANSI/AAMI PB70 standard recognized by the FDA.
| Type of PPE | Feature Tested | Standard Designation | Sub headings | Description | Applicability |
| Gowns | Liquid Barrier Performance | AAMI PB70:2012 | Classifies a gown’s ability to act as a barrier to penetration by liquids or liquid-borne pathogens based on four levels.
The critical protective zones for surgical and non-surgical gowns are defined differently by the standard. While the critical zones designate different protective areas for the different gowns, the levels of protection are the same for both surgical and non-surgical gowns |
Liquid barrier performance is not related to the strength of the material.
This standard references several other standards |
|
| Level 1 |
|
basic care, standard hospital medical unit | |||
| Level 2 |
|
Blood draw from a vein, Suturing, Intensive care unit, Pathology lab | |||
| Level 3 |
|
Arterial blood draw, Inserting an IV, Emergency Room, Trauma | |||
| Level 4 |
|
Pathogen resistance, Infectious diseases (non-airborne), Large amounts of fluid exposure over long periods |
Conformance with recognized consensus standards is voluntary for a medical device manufacturer. A manufacturer may choose to conform to applicable recognized standards or may choose to address relevant issues in another manner.
