Description
Basic Information
- NIOSH Approved: N95
- FDA Cleared for use as surgical mask
- Helps protect against certain airborne biological particles
- Fluid resistant and disposable
| 3M™ Health Care Particulate Respirator and Surgical Mask 1860… N95 |
| Item No. | 1860 |
| Brand | 3M |
| SKU: | 70070612364 |
| UPC: | 50707387419429 |
| Shape/Function | Cup shaped without valve and active carbon |
| Standard | NIOSH N95 |
| FDA | Cleared for use as surgical mask |
| Style | Head strap |
| Aerosol Type | Non-Oil |
| Elastic head band | Braided Comfort Strap |
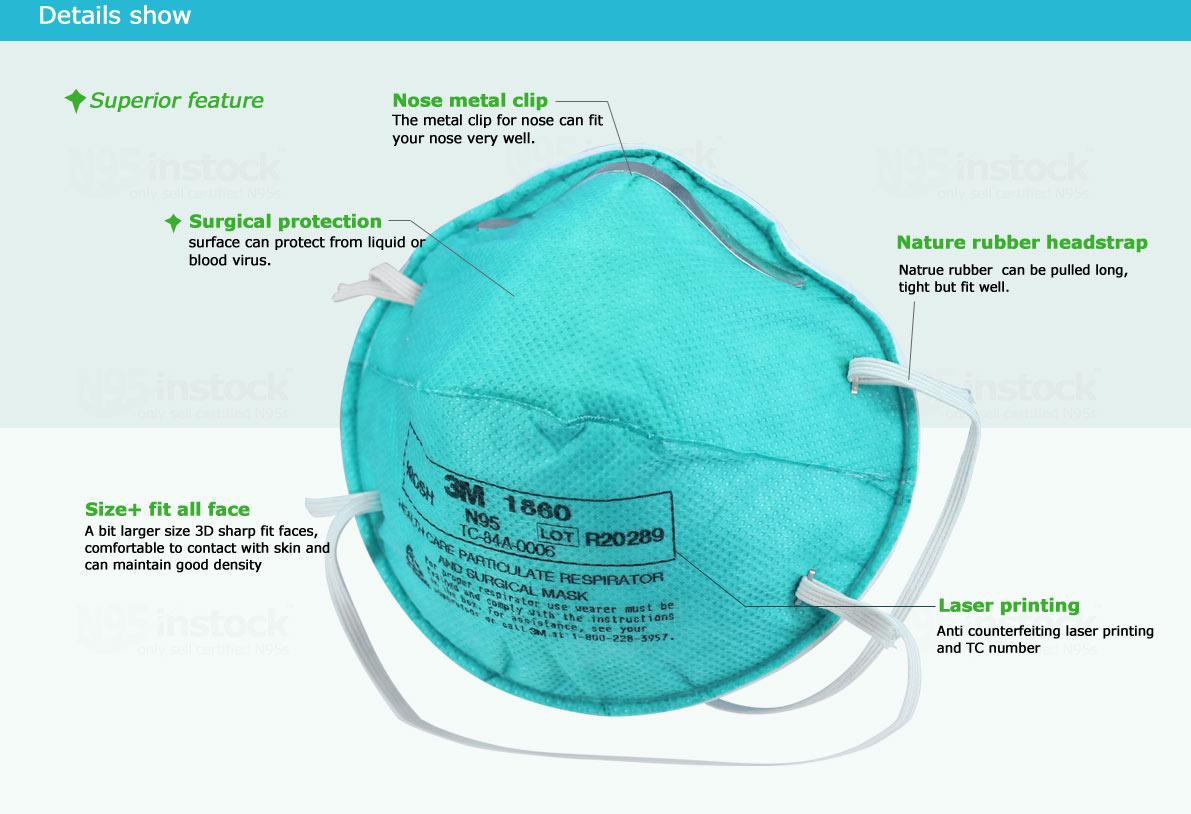
| Adjustable nose clip | Aluminum 90mm*5mm |
| Soft foam nosepiece | PVC nose pas, gray |
| Fluid Resistant | ASTM F1862 (Yes) |
| Dimensions | 145*120*53mm± |
| Layers/material | 3 layers: PP spunbond outer layer, PP melt blown high filtration layer, PP spunbond outer layer and inner layer. |
| Outer layer | PP spunbond |
| Second layer | Electrostatic high-efficiency filter material |
| Third layer | Meltblown non-woven fabric |
| Inner Layer | PP needle punched cotton non-woven |
| Packaging | 120 pieces/case |
| Weight/pc | 13.2g ± 0.3g |
| Application | Medical, Healthcare Respirator, Agricultural Production, Airborne Biological Particles, Bagging, Dry Chemical Handling, Emergency & Pandemic Preparedness, Foundry Operations, Grinding, Petrochemical Manufacturing, Processing of Minerals, Sanding, Textile Operations |
| Recommended Industry | Health Care |
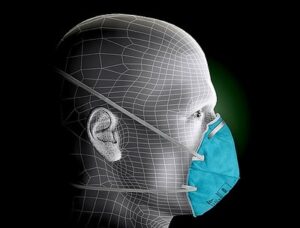
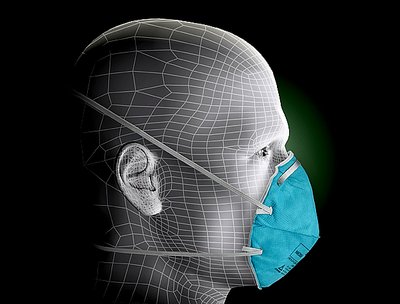
The 3M 1860 is a higher grade N95 among all nine NIOSH standards. The N95 mask filters at least 99% of airborne particles, is fluid resistant, but is not resistant to oil. With its specially designed “facial seal encasement”, this model fully meets with NIOSH approval requirement for breathing resistance and filtration efficiency at the N95 level.
This healthcare respirator is designed to help provide respiratory protection for the wearer. It meets CDC guidelines for Mycobacterium tuberculosis exposure control. As a disposable particulate respirator, it is intended to help reduce wearer exposure to certain airborne particles including those generated by electrocautery, laser surgery, and other powered medical instruments. As a surgical mask, it is designed to be fluid resistant to splash and spatter of blood and other infectious materials.
Features include:
- NIOSH approved N95
- Meets CDC guidelines for Mycobacterium tuberculosis exposure control
- FDA cleared for use as a surgical mask
- 99% BFE (Bacterial Filtration Efficiency) according to ASTM F2101
- Fluid resistant according to ASTM F1862
- Respirator contains no components made from natural rubber latex
- Collapse resistant cup shape design
- Braided headbands, cushioning nose foam, and light weight construction for comfortable wear
Suggested settings and applications: Operating Rooms, Clinics, TB Wards, Patient Care, Labor and Delivery, Infection Control Practices, Laboratory, emergency or pandemic preparedness planning, stockpiling, etc.
WARNING: These respirators help reduce exposures to certain airborne contaminants. Before use, the wearer must read and understand the User Instructions provided as a part of the product packaging. In the U.S., a written respiratory protection program must be implemented, meeting all the requirements of OSHA 1910.134 including training, fit testing and medical evaluation. In Canada, CSA standards Z94.4 requirements must be met and/or requirements of the applicable jurisdiction, as appropriate. Misuse may result in sickness or death. For proper use, see package instructions.
Product Description
- NIOSH Approved: N95
- FDA Cleared for use as surgical mask
- Helps protect against certain airborne biological particles
- Fluid resistant and disposable
* High filter material with 99% efficiency
* Wider edge for secure facial seal
* Adjustable button design is suitable for different face shapes
* Braided two-strap design
Cautions and Limitations
General Cautions and Limitations for All Part 84A Approvals – Non-powered Air-Purifying Particulate Filter Respirators. These limitations are by no means all-inclusive.
- Not for use in atmospheres containing less than 19.5 percent oxygen.
- Not for use in atmospheres immediately dangerous to life or health.
- Do not exceed maximum use concentrations established by regulatory standards. Failure to properly use and maintain this product could result in injury or death.
- Failure to properly use and maintain this product could result in injury or death.
- All approved respirators shall be selected, fitted, used, and maintained in accordance with MSHA, OSHA, and other applicable regulations.
- Never substitute, modify, add or omit parts. Use only exact replacement parts in the configuration as specified by the manufacturer.
- Refer to User’s Instructions, and/or maintenance manuals for information on use and maintenance of these respirators.
- NIOSH does not evaluate respirators for use as surgical masks.


Resources
Brochures
- 3M™ Healthcare Particulate Respirator & Surgical Mask 1860 & 1860S Series Brochure (PDF, 336.4KB)
- Health Care Brochure (PDF, 12.1MB)
- 3M™ Full Line Mask and Respirator Brochure (PDF, 1.9MB)
Data Sheets
- 1860-1860S User Instructions (PDF, 233.8KB)
- 3M™ Health Care Particulate Respirator and Surgical Masks Storage Conditions and Shelf Life FAQ (PDF, 142.6KB)
- Quick Reference Guide – Disposable Respirators (PDF, 4.9MB)
- Tech Spec 3M Healthcare Particulate Respirator and Surgical Mask 1860, N95 (PDF, 182.7KB)
- 1860 & 1860S Package Appearance, Shelf Life Markings (PDF, 495.8KB)
Instructions
- 3M™ Healthcare N95 Respirator 1860, 1860S, 1870 Instructions Sheet (PDF, 180.9KB)
- Health Care Particulate Respirator and Surgical Mask 1860/1860S User Instructions (PDF, 232.2KB)
- Common Questions – 3M N95 Particulate Respirator/SurgMask (PDF, 18.0KB)
Letters
Posters
- 1860 and 1860S Wear it Right Poster (PDF, 379.0KB)
- Health Care Respirator 1860/1860S – WearItRightPoster – English (PDF, 233.7KB)
Shipping
FedEx/DHL/UPS/TNT/USPS for samples, Door-to-Door
By Air or by Sea for batch goods, EXW/FOB/CIF/DDP available
Customers specifying freight forwarders or negotiable shipping methods
Delivery Time: 1-2 days for samples; 7-14 days for batch goods.
Why choose us?
* 7*24 online EMAIL/Trade Manager/Wechat/WhatsApp service!
* We are a factory manufacturing disposable dust masks, best production flexibility, best quality control, best Service.
* 100% QC inspection Before Shipment.
* NIOSH / CE / Benchmark listed respirator masks, competitive price.
* Daily capacity over 2 million pieces for NIOSH N95 mask
* First NIOSH N95 mask approved in 2012, successfully passed the 4th NIOSH site audit in 2020
* On the white list of China non-medical export and USA FDA EUA







Reviews
There are no reviews yet.